This is the third article in a special Forkast.News series on blockchain, Covid-19 and the future of healthcare. To learn more about this series, please see the full episode list here.
——————————————————————————
While 2020 will go down in history as a year to skip, thus far 2021 is one of greater promise and optimism given the regulatory approval and rollouts of the Pfizer/BioNTech, Moderna and Oxford/AstraZeneca vaccines, which offer between 90% and 95% protection against Covid-19. Yet, with the end seemingly in sight, novel variant strains of the virus have been popping up, some of which can side-step the current vaccines.
AstraZeneca’s vaccine rollout has been halted in South Africa, as a study has indicated it does not provide sufficient protection against the new B.1.351 variant strain. Pfizer/BioNTech’s vaccine, however, works just as well. Mutations, supply chain issues, and confusion over “which vaccine to take” is giving rise to vaccine skepticism.
Innovative technologies have a part to play to assuage these anxieties. Blockchain, for example, can help combat the skepticism, but also has the potential to revolutionize drug and vaccine development. Over the past decade, vaccine trials have become increasingly complex, digitalized and data-heavy, with data generated from a growing number of different sources — sensors, wearables and mobile devices. Gone are the days of “big-pharma” using clinical research assistants to log patient data down on paper.
However, any data that gets logged electronically is susceptible to human error and data manipulation. Blockchain technology offers a means to ensure data verification, protection, integrity, auditability and traceability in vaccine trial design and data management.
Fastest ever vaccines from concept to reality
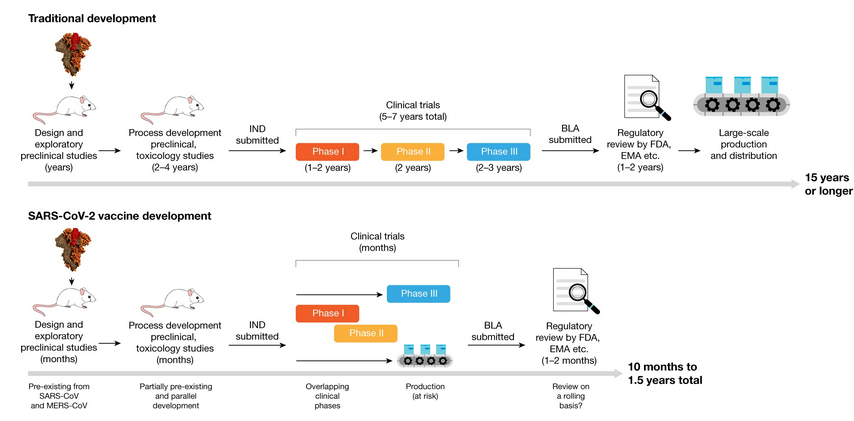
The new Covid vaccines are the fastest ever to go from concept to reality. It took just 10 months to navigate the pathway to success, a process that normally spans up to 10 years. Furthermore, historically, less than 10% of all drug or vaccine candidates make it through all the stages of development and trials to regulatory approval and market entry. In the pharmaceutical industry, the fully loaded costs for drug development to pre-approval average an estimated US$2.6 billion.
Vaccine development for Covid-19 benefited from extensive research and development carried out on previous Coronavirus epidemics — SARS and MERS. This, plus the prodigious amount of capital — including the US$18 billion pumped into “Operation Warp Speed” by the U.S. Government, and US$1.7 billion from The Gates Foundation, – plus the use of Fast Track and Emergency Regulatory Codes, meant the discovery phase could be legitimately by-passed.
Phase trials began unencumbered, with several Phase 3 clinical trial stages running in parallel by the middle of 2020. At the same time, vaccine producers started large-scale production of several vaccine candidates, even before the outcomes of the Phase 3 trials were known.
However, “Operation Warp Speed,” took place against a very unpleasant political backdrop, whereby even simple protective behavior such as mask-wearing became highly politicized. Paranoia, fake news, political extremism and overall fear were reflected in polls, with many Americans having reduced faith in “rushed” Covid-19 vaccines.
New age of clinical trial data; new age of conspiracy
To the mix of digital technology and innovation, such as wearables and biometric patches that track side effects, blood pressure, temperature, heart rate and so forth, add in blockchain technology and what then becomes possible is a “new age” of digital clinical trials. Blockchain can have a global impact on clinical research because it allows for independent, objective and sentinel data tracking. Patients can be remotely and confidentially enrolled, with each individual trial tracked in real time, privately on the blockchain, ensuring the safety and privacy of participants while shortening trial timelines.
Blockchain can dramatically reduce the human workload and the risk of human error. All these factors speed data collection, increase accuracy, encourage data sharing and promote regulatory compliance. The data becomes immutable, and any changes would have to be approved by more than 51% of all users across a network. It can also track and keep tally of who has accessed which part of the datasets, thus creating an audit trail that improves privacy and data security.
How the vaccine works — safety and distribution
The Pfizer/BioNTech, Oxford/Astra-Zeneca and Moderna vaccines are all mRNA vaccines based on the SARS-CoV-2 “spike protein,” the mechanism used by the virus to gain entry into human epithelial cells. mRNA stands for “messenger ribonucleic acid” and it literally is that — messenger information. There is zero biological possibility of contracting Covid-19 from vaccines using mRNA technology. It does not change a patient’s DNA, and it cannot integrate into their genome or alter the genetics.
Scientists have used molecular “cutting tools” to cut out a key piece of the virus structure and convert it into a data package. The message guides the vaccine user’s immune system through the process of developing an anti-Covid-19 immune response, but without having to endure the actual disease. As with all vaccines, the immune system responds to, or “sees” tiny components of the infection, which induces the protective response, but this mimicry can be reflected in the low-grade symptoms experienced post-vaccination. There can also be pain at the injection site, and perhaps a general feeling of malaise for a week or so.
Historically, most vaccines are developed in combination with booster substances to help the immune system elicit a stronger response. These boosters are called “adjuvants.” For the mRNA vaccines, naked mRNA cannot easily enter cells and needs help to get into the skin cells. The delivery vehicle for the Pfizer/BioNTech and Moderna vaccines is called a lipid nanoparticle (LNP). LNPs are microspheres that surround the mRNA, and their “oiliness” help the mRNA to slip into cells easily, delivering their payload of mRNA.
The approved Covid-19 vaccines are given in two doses, a few weeks apart. With the first injection, your immune cells become “primed,” or “see” the spike protein for the first time. It takes some time for the first responder immune cells in the skin, muscle, blood and lymph nodes, to communicate with T cells and B cells — T cells are part of the immune response to a viral infection, while B cells generate and pump out the life-saving antibodies that protect against Covid-19.
By the time of the second dose, the immune system has already had a “heads-up” and has “memory” from the first shot. This second shot literally “boosts” Covid-19 immunity up to the up to 90 to 95% protection shown in clinical trials. Not returning for the second boost will lead to inadequate immunity to Covid-19.
Serendipity — mixing and matching vaccine brands and booster shots
The unprecedented “mixing and matching” different brands of Covid-19 vaccines and booster shots, and lengthening the time between the shots, emerged out of supply chain problems and was not part of the plan. It was not how the pharmaceutical companies designed, trialled and gained the vaccines’ approval for use.
However, with more than 10 million people now vaccinated in the U.K., data has emerged, most serendipitously, that mixing and matching shots, plus delaying booster shots has conferred improved protection against Covid-19. The U.K. Government Taskforce is ploughing £7 million, or about US$9.7 million, into a study known as “COVID-19 heterologous Prime Boost Study,” which aims to evaluate the four different combinations of vaccination. The U.K. government has hailed the trial as a world first.
In another first, the mRNA technology used in both the Pfizer/BioNTech and Moderna’s vaccine platforms can easily be adapted to target new virus strains, with both groups working on developing rapid “new boosters” as add-ons to the initial jab. As with influenza, which mutates very rapidly, and requires an annual booster, it is likely that Covid vaccines will also need to be seasonally updated.
Vaccine manufacture and distribution as critical as discovery itself
However, the manufacturing and equitable distribution of the vaccines is going to be as critical as the scientific discovery itself. Already, in the early weeks of the rollouts, weak spots in the supply and distribution chains are being highlighted, resulting in slower than expected vaccinations administered, squabbles between governments and continents over shipments, and vaccine waste.
Efficiently producing, flawlessly distributing, equitably delivering and storing millions of tiny vials at the right temperature is a momentous task — and something that can be greatly facilitated by leveraging blockchain technology. Managed properly, this momentous task will go down as a historic achievement, show-casing unparalleled speed-to-market for critical vaccines that hopefully mark our path out of the pandemic and toward a life akin to the one we once knew.




