While the Pfizer (PFE) vaccine is the first of the vaccines expected to get regulatory approval this year, its distribution may be limited considering its extremely low storage and distribution temperature of minus 70 degrees Celsius and below.
The BNT162b2 vaccine, jointly developed by the duo of Pfizer Inc (NYSE: PFE) and BioNTech SE (NASDAQ: BNTX) is now being administered in the United Kingdom. According to a Reuters report, the vaccine rollout and administration comes following the Emergency Use Authorization (EUA) granted by the UK’s Medicines and Healthcare products Regulatory Agency (MHRA).
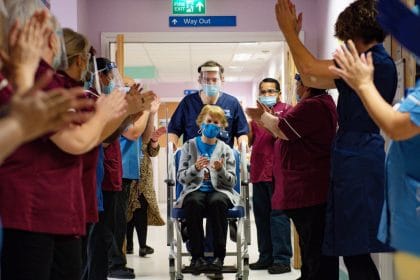
Per the reports, the first person in the world post-trial to receive the long-anticipated COVID-19 vaccine is 90 years old grandmother Margaret Keenan from Northern Ireland. Margaret received the BNT162b2 vaccine shots at her local hospital in Coventry by nurse May Parsons. With just a week to her 91st birthday, Margaret noted that she “feels so privileged to be the first person vaccinated against COVID-19.”
The UK now ranks as the first Western country to immunize its citizens against the novel Coronavirus, a disease which broke out in China about a year ago and has killed over 61,000 people in the United Kingdom and 1.5 million people worldwide. The UK is now ahead of the United States, and the European Union in administering the COVID-19 vaccine.
Today the first vaccinations in the UK against COVID-19 begin. Thank you to our NHS, to all of the scientists who worked so hard to develop this vaccine, to all the volunteers – and to everyone who has been following the rules to protect others. We will beat this together. https://t.co/poOYG1vHQe
— Boris Johnson (@BorisJohnson) December 8, 2020
While the Pfizer (PFE) vaccine is the first of the vaccines expected to get regulatory approval this year, its distribution may be limited considering its extremely low storage and distribution temperature of minus 70 degrees Celsius and below.
PFE stock closed up 2.26% to $41.25 Monday, a move that outperformed key market indices including the S&P 500 Index (INDEXSP: .INX) and the Dow Jones Industrial Average (INDEXDJX: .DJI) which dropped 0.19% and 0.49% respectively.
Potential Pfizer (PFE) Vaccine Brawl with the US
Despite the United Kingdom taking the lead in approving and rolling out the Pfizer vaccines, the United States is expected to also grant the EUA for the vaccine any moment from now. While the United States comes off as one of the nations with the biggest deal with Pfizer and its partner BioNTech, the country may not be getting enough supply of the vaccines beyond the 100 million doses in its contract.
As reported by the Washington Post, there are fears among some government officials that the US may not have enough vaccines to immunize the majority of its population. This fear is despite claims that the US has deals with other pharmaceutical companies working on the COVID-19 vaccine. The deals with these other parties have been duly noted that it will be insufficient to meet the needs of the US population.
The Pfizer vaccine is expected to be administered in two doses which implies that the 100 million doses ordered will only serve 50 million people. From the current demand for Pfizer’s vaccines, the US may likely not get additional doses until late June or July.
Benjamin Godfrey is a blockchain enthusiast and journalists who relish writing about the real life applications of blockchain technology and innovations to drive general acceptance and worldwide integration of the emerging technology. His desires to educate people about cryptocurrencies inspires his contributions to renowned blockchain based media and sites. Benjamin Godfrey is a lover of sports and agriculture.